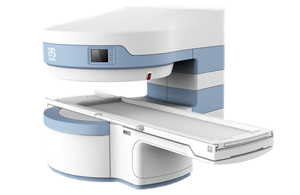
Resonador Magnético 0.3T OPENMARK III: Innovación y Comodidad en Diagnóstico por Imagen
El Resonador Magnético 0.3T OPENMARK III es una avanzada herramienta de diagnóstico por imagen, desarrollada en 2004. Aprobado por la FDA y la CE, este resonador de imán permanente combina tecnología de punta con un diseño ergonómico para proporcionar resultados precisos y eficientes en una amplia variedad de aplicaciones clínicas.
Características Destacadas del Resonador Magnético 0.3T OPENMARK III
- Imán Permanente de 0.3T: El OPENMARK III está equipado con un imán permanente de 0.3 Tesla, que garantiza una excelente calidad de imagen mientras reduce los costos operativos en comparación con los sistemas de campo alto.
- Diseño Abierto en C: El diseño de imán abierto en C del OPENMARK III permite la visión más amplia posible, mejorando significativamente la comodidad del paciente y reduciendo la ansiedad, especialmente en pacientes con claustrofobia o limitaciones físicas.
- Imágenes Rápidas y de Alta Calidad: Gracias a su tecnología avanzada, el OPENMARK III proporciona imágenes rápidas y de alta calidad, esenciales para diagnósticos precisos y confiables.
- Protocolos Preestablecidos y Aplicaciones Avanzadas: El OPENMARK III ofrece numerosos protocolos preestablecidos de exploración, así como aplicaciones avanzadas como la difusión de imágenes, imágenes ultra-rápidas, angiografía y exploración de apnea. Estas funciones permiten a los profesionales médicos realizar una amplia gama de estudios con facilidad y precisión.
- Aprobaciones Internacionales: La aprobación por la FDA y la certificación CE aseguran que el OPENMARK III cumple con los más altos estándares de calidad y seguridad, proporcionando tranquilidad tanto a los profesionales de la salud como a los pacientes.
Beneficios Clave
- Calidad de Imagen Superior: Con su avanzada tecnología, el OPENMARK III proporciona imágenes claras y detalladas, permitiendo una visualización precisa de las estructuras internas del cuerpo.
- Mayor Comodidad para el Paciente: El diseño abierto en C facilita el acceso y mejora la comodidad del paciente durante el procedimiento, reduciendo la ansiedad y mejorando la experiencia general del paciente.
- Eficiencia Operativa: La tecnología de imán permanente y el diseño optimizado del OPENMARK III aseguran una operación eficiente y rentable, ideal para clínicas y hospitales que buscan maximizar su rendimiento sin comprometer la calidad.
- Versatilidad en Aplicaciones Clínicas: Adecuado para una amplia gama de aplicaciones clínicas, desde exploraciones ortopédicas hasta evaluaciones neurológicas, el OPENMARK III es una solución versátil y fiable para diversas necesidades de diagnóstico por imagen.
- Seguridad y Fiabilidad: La aprobación por la FDA y la certificación CE garantizan que el OPENMARK III cumple con estrictos estándares de seguridad y calidad, ofreciendo fiabilidad y confianza en cada examen.
Aplicaciones Clínicas
- Exploraciones Ortopédicas: Obtén imágenes detalladas de articulaciones, huesos y tejidos blandos, esenciales para el diagnóstico y tratamiento de afecciones ortopédicas.
- Evaluaciones Neurológicas: Imágenes claras y precisas del cerebro y la médula espinal, fundamentales para el diagnóstico de enfermedades neurológicas.
- Diagnósticos Abdominales y Pélvicos: Facilita la exploración de la cavidad abdominal y pélvica, proporcionando información crucial para el diagnóstico de diversas patologías internas.
- Estudios Vasculares: Realiza angiografías y otros estudios vasculares con alta precisión, mejorando la detección y evaluación de enfermedades vasculares.
El Resonador Magnético 0.3T OPENMARK III es una herramienta indispensable para cualquier instalación médica que busque ofrecer diagnósticos precisos y eficientes. Con su imán permanente de 0.3 Tesla, diseño abierto en C, y aplicaciones avanzadas, el OPENMARK III se posiciona como una solución avanzada y confiable en el campo de la resonancia magnética.
Descubre cómo el OPENMARK III puede transformar tu práctica médica, proporcionando diagnósticos de alta calidad y mejorando la experiencia del paciente. Para más detalles, contact us here.